

Translating complex clinical trials into compelling resume content can feel like running a difficult protocol. Many Clinical Research Coordinators get stuck trying to balance technical expertise with clear, recruiter-friendly language.
Are you struggling to showcase both your scientific knowledge and project management abilities? Your resume needs to demonstrate how you bridge the gap between protocols and people while highlighting your track record of successful trial management.
Resume Target specializes in helping Clinical Research Coordinators transform their experience into powerful career stories. We know how to present your trial management expertise, regulatory knowledge, and patient care skills in a way that gets noticed by hiring managers.
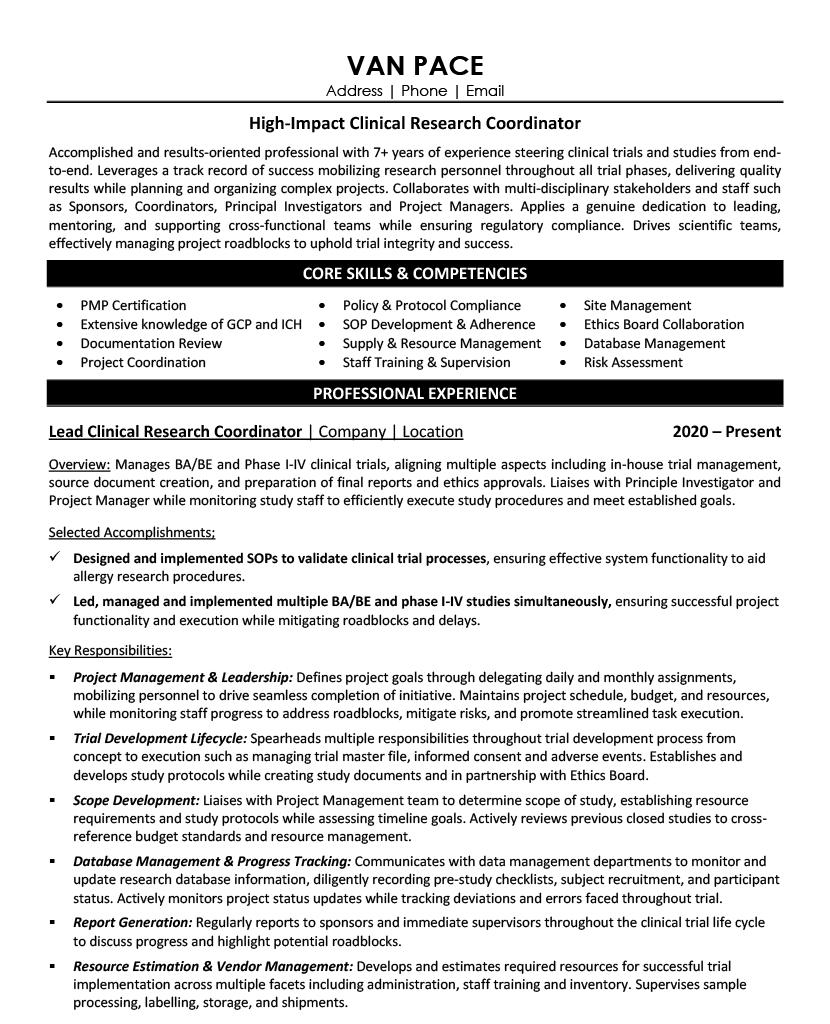

At the intersection of groundbreaking medical discoveries and patient care, Clinical Research Coordinators serve as the vital bridge that transforms scientific theories into life-changing treatments, managing critical aspects like daily operational support of clinical trials and maintaining documentation compliance.
Working alongside Principal Investigators and research teams, you'll orchestrate complex clinical trials from start to finish - recruiting participants, ensuring proper informed consent, coordinating study visits, and meticulously documenting every step to maintain the integrity of potentially revolutionary medical research.
Whether you're just starting out or looking to advance your career, the field of clinical research coordination offers diverse opportunities for growth, from specializing in specific therapeutic areas to advancing into clinical research management roles where you'll shape the future of medical research.
Let's talk about the exciting earning potential in Clinical Research Coordination! Your career path can take you from entry-level positions all the way to senior roles, with compensation growing substantially as you gain experience and expertise. And guess what? The field offers plenty of room for advancement as you develop your skills and take on more responsibilities.
Figures from: Clinical Research Coordinator Salary Guide
Starting as a Clinical Research Coordinator opens doors to rewarding career growth in healthcare research. With the right education and experience, you can advance to senior positions earning $90,000+.
Beyond basic coordination abilities, mastering these key skills will accelerate your advancement in clinical research leadership.
- Good Clinical Practice (GCP) certification and regulatory compliance - Clinical data management and analysis expertise - Protocol development and implementation - Cross-functional team leadership and communicationBreaking into clinical research coordination starts with combining healthcare experience, research knowledge, and certification - with entry-level positions offering valuable stepping stones to full CRC roles.
To advance in this field, you'll need to develop essential skills including regulatory knowledge and Good Clinical Practice (GCP) compliance, which form the foundation of successful clinical research coordination.
Requirements from ACRP Certification
From biotech hubs to healthcare centers, CRC opportunities are thriving in both coastal and inland regions.
Figures from Reddit Clinical Research Community
Struggling to showcase your clinical trial management expertise, patient coordination skills, and research protocol knowledge in a way that catches a hiring manager's eye? This comprehensive, section-by-section guide will walk you through creating a Clinical Research Coordinator resume that highlights your most impressive achievements and demonstrates your ability to drive successful research studies.
If you're like most Clinical Research Coordinators, condensing years of complex trial management, regulatory compliance, and patient care experience into a few powerful sentences can feel overwhelming.
While you excel at organizing multi-site studies and maintaining meticulous documentation, translating these specialized skills into a compelling summary that catches a hiring manager's attention requires a different kind of precision - one that showcases both your technical expertise and your ability to drive successful trial outcomes.
How would you characterize your overall approach to managing clinical trials and what distinguishes you from other Clinical Research Coordinators in terms of methodology and style?
Reason: This helps establish your professional identity and preferred working style, allowing you to showcase your unique value proposition in clinical research management. It sets the tone for how you approach the complex demands of trial coordination.
What combination of therapeutic areas and study phases have you worked with throughout your career that best represents your expertise as a Clinical Research Coordinator?
Reason: This question helps you articulate your breadth of experience across different types of clinical trials, demonstrating your versatility and depth of knowledge in specific therapeutic areas that employers may be seeking.
How would you describe your strongest professional attributes in terms of regulatory compliance, patient interaction, and cross-functional team collaboration?
Reason: This helps you identify and articulate the core competencies that make you effective in the three most critical areas of clinical research coordination. It allows you to present a well-rounded professional profile that addresses key stakeholder concerns.
As a Clinical Research Coordinator, you need to showcase both your scientific expertise and your ability to manage complex clinical trials while maintaining regulatory compliance.
Your resume should highlight technical skills like protocol implementation and IRB submissions, alongside essential day-to-day abilities such as patient recruitment, data management in EDC systems, and cross-functional team coordination.
Showcase your clinical research expertise by organizing your experience into three powerful sections: a concise role overview highlighting your research environment, measurable achievements that spotlight your study management success, and core responsibilities that demonstrate your protocol and regulatory compliance skills.
Many Clinical Research Coordinators struggle to effectively showcase their dual expertise in both patient care and complex protocol management. Transform your experience into compelling metrics by connecting your coordination skills to successful trial completions, regulatory compliance rates, and participant retention outcomes.
The responsibilities section demonstrates how Clinical Research Coordinators manage complex clinical trials while ensuring patient safety and data integrity. Your role bridges the gap between medical research and practical implementation, directly impacting the development of new treatments and therapies.
Your education and certifications demonstrate your expertise in clinical research protocols and human subject protection. Lead with your highest relevant degree and current SOCRA or ACRP certification, then list any specialized training in Good Clinical Practice (GCP) or research compliance.
Now that you've built a strong foundation using Resume Target's proven resume writing guidelines, you're ready to transform your CV into a powerful tool for landing your ideal Clinical Research Coordinator position.
While many candidates stop at customizing their cover letter, successful Clinical Research Coordinators know that personalizing their resume for each position is what truly sets them apart in this competitive field.
By strategically incorporating specific keywords and requirements from each job posting, your tailored resume will not only sail through ATS screening systems but will also immediately show hiring managers how perfectly your clinical research experience aligns with their needs.
Ready to turn your resume into your secret weapon? Let's make every application count by crafting a targeted resume that proves you're the Clinical Research Coordinator they've been searching for!
Don't let a lack of direct experience hold you back from launching your Clinical Research Coordinator career!
Your academic background in life sciences, healthcare, or related fields, combined with any research projects or internships, creates a strong foundation for entering this dynamic field.
Focus on highlighting your understanding of research protocols, data management skills, and any relevant laboratory or clinical exposure.
For more detailed guidance on structuring your entry-level resume, check out our Student Resume Writing Guide to ensure you're showcasing your potential effectively.
Your summary section is your chance to showcase your academic foundation in healthcare, research experience from internships, and any relevant clinical exposure you've gained during your studies.
Focus on highlighting your understanding of research protocols, data management skills, and passion for advancing medical knowledge through well-coordinated clinical trials.
"Detail-oriented and methodical Clinical Research Coordinator with hands-on experience through university research projects and healthcare internships. Demonstrated expertise in IRB documentation, data collection protocols, and HIPAA compliance while assisting with three clinical studies at University Medical Center. Proficient in REDCap, Microsoft Office Suite, and clinical data management systems. Seeking to leverage strong organizational abilities and research background to contribute to meaningful clinical trials that advance patient care."
Now's your chance to showcase the academic foundation that prepared you for coordinating vital medical studies and managing research protocols!
Transform your educational background into compelling content by highlighting relevant coursework like Clinical Trial Management and Research Ethics, plus any capstone projects where you developed protocols or managed study data.
unavailableRelevant Coursework: Clinical Research Methods | Biostatistics | Research Ethics | Clinical Trial Management | Data Management Systems | Healthcare Regulations
Key Projects:
Clinical Trial Database Implementation: Developed and executed a mock clinical trial database system to track patient enrollment and outcomes for a diabetes medication study.
Multi-Site Study Coordination Simulation: Led a team of 4 students in simulating the coordination of a multi-center clinical trial for cardiovascular research.
Leverage your academic background, internship experiences, and clinical research certifications to create a compelling skills section that showcases your readiness to coordinate vital medical studies and manage research protocols.
As an entry-level Clinical Research Coordinator, your foundation in research methodology and regulatory compliance positions you well for a field that continues to grow with the expanding needs of pharmaceutical and medical research industries.
Let's face it - translating your complex trial management experience and intricate regulatory knowledge into a clear, compelling story can feel overwhelming, especially when you're juggling multiple protocols and patient care responsibilities.
At Resume Target, we specialize in crafting resumes for biotech professionals just like you, having helped countless Clinical Research Coordinators showcase their expertise in protocol adherence, patient recruitment, and data management.
With clinical trials becoming increasingly competitive and complex, your resume needs to stand out more than ever - let's transform your experience into a powerful career story that hiring managers can't ignore. Schedule your free consultation today.
Impress any hiring manager with our Biotechnology resume writing service. We work with all career levels and types of Biotechnology professionals.
Learn More → Biotechnology Resume Writing Services