Clinical Research Coordinator
Resume Sample
A real resume example showing how we transform trial management and regulatory expertise into proof employers trust
Being qualified isn't enough — you need to be the obvious choice.
We fix your resume with one conversation
What Makes a Strong Clinical Research Coordinator Resume?
A Clinical Research Coordinator resume must showcase trial phase experience, regulatory compliance, and Ethics Board collaboration. Employers scan for GCP/ICH knowledge, SOP development, and multi-study management. This sample demonstrates how interview-extracted achievements showcase clinical research excellence across therapeutic areas.
Why Do Clinical Research Coordinator Resumes
Get Rejected?
Most clinical research coordinator resumes get rejected not because of ATS software, but because they don't prove you're better than the other 73 applicants. Generic bullets like "managed construction projects" don't differentiate you — quantified achievements do.
See how we transform generic statements into interview-winning proof:
Led, managed and implemented multiple BA/BE and phase I-IV studies simultaneously, ensuring successful project functionality and execution while mitigating roadblocks and delays."
This shows multi-study leadership: BA/BE (bioavailability/bioequivalence) and Phase I-IV shows full trial spectrum. "Simultaneously" demonstrates concurrent project management. SOP development shows process leadership. Allergy research specificity shows therapeutic area experience. Roadblock mitigation shows problem-solving capability.
Managed and directed implementation of multi-center clinical trials at Company (University). Championed end-to-end trial elements from study planning to final study deliverables while collaborating with dentists, orthodontists and graduate students."
This shows research leadership: "from scratch" demonstrates building capability. Research and data templates shows standardization. Advising students and professors shows training expertise. Multi-center trials shows complexity management. Dentists and orthodontists shows therapeutic area breadth. End-to-end ownership shows comprehensive accountability.
Ethics Board Collaboration: Prepares ethics documentation for board review and approval, highlighting study intentions and implications on voluntary participants to create informed consent forms.
Regulatory Compliance Management: Manages staff compliance to established regulatory protocols and SOPs throughout study to effectively mitigate potential health, safety, and ethical risks."
This shows complete lifecycle ownership: concept to execution covers full span. Trial master file, informed consent, adverse events shows regulatory knowledge. Ethics Board partnership shows compliance focus. Informed consent documentation shows patient safety priority. Health, safety, and ethical risk mitigation shows comprehensive compliance management.
How Do Pharmaceutical Resume Writers Transform a Clinical Research Coordinator Resume?
Professional resume writers transform clinical research coordinator resumes by analyzing job postings for required keywords, extracting specific achievements through targeted questions, quantifying impact with dollar values and percentages, and positioning you as the solution to employer problems.
We Analyze Clinical Research Coordinator Job Postings
We identify exactly what hiring managers search for:
- Budget management and cost control requirements
- Schedule recovery and timeline management skills
- Site safety compliance and OSHA standards
- Subcontractor coordination and vendor management
We Extract Your Achievements
Our 1-on-1 interview uncovers:
- Project values and budgets you've managed
- Team sizes and subcontractors you've coordinated
- Problems you've solved that others couldn't
- Metrics you didn't think to track or quantify
We Quantify Your Impact
We find the numbers that prove ROI:
- Dollar values of projects completed on time
- Percentage of schedule improvements achieved
- Cost savings from value engineering decisions
- Safety record improvements and incident reductions
We Position You as the Solution
Your resume proves you solve employer problems:
- Delivering projects on time despite site challenges
- Managing subcontractors and maintaining quality
- Controlling costs while meeting specifications
- Leading teams through complex project phases
Listen to a Real Resume Interview
Hear how our writers extract clinical research achievements through targeted questions.
What Does a Clinical Research Coordinator Resume Interview Look Like?
A clinical research coordinator resume interview is a conversation where our writer asks targeted questions about your projects, probes for specific details, and extracts achievements you'd never think to include.
Designed and implemented SOPs to validate clinical trial processes, ensuring effective system functionality to aid allergy research procedures.
Led, managed and implemented multiple BA/BE and phase I-IV studies simultaneously, ensuring successful project functionality and execution while mitigating roadblocks and delays.
Every bullet on this resume was created through this same process.
Schedule Your InterviewHave questions? 1-877-777-6805
Watch How We Transformed Khoi's Resume
See how our interview process uncovered achievements that generic templates miss.
Get Your Resume Transformed
What a Clinical Research Coordinator Resume Example That Gets Interviews Looks Like
A complete clinical research coordinator resume is typically 2 pages and includes a professional summary, core competencies, detailed work experience with quantified achievements, education, and certifications. Here's both pages of an actual resume created through our interview process.
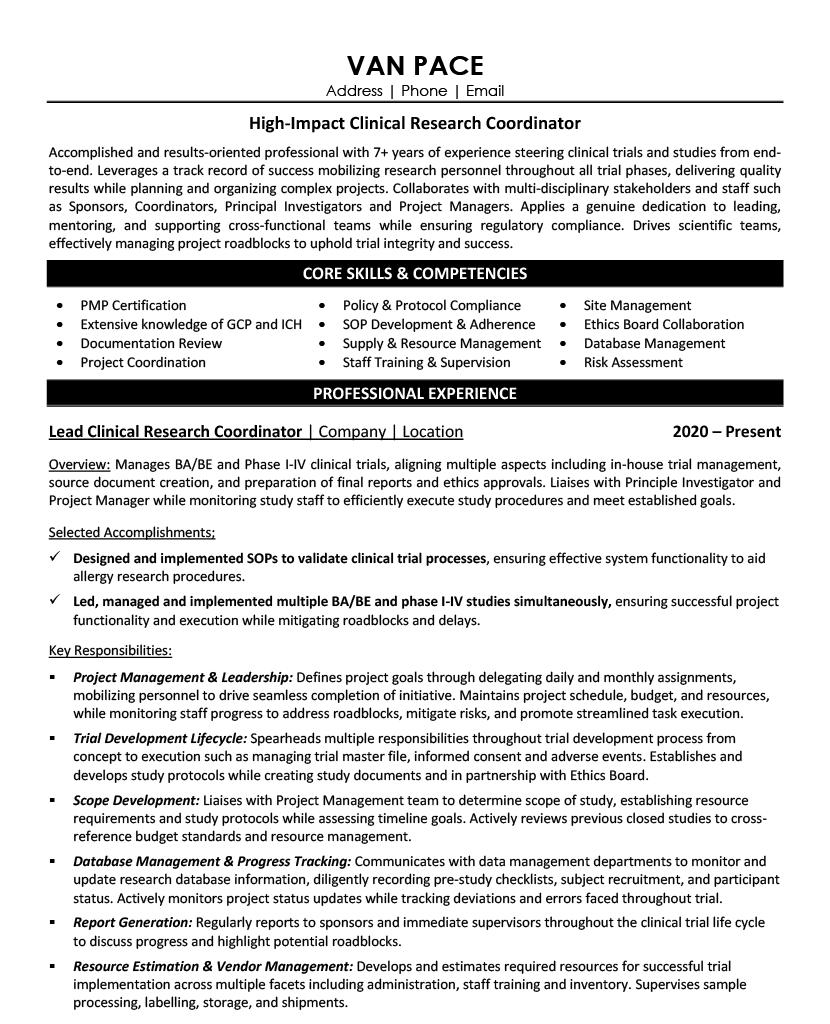

Which Clinical Research Coordinator Resume Example
Do You Need?
The clinical research coordinator resume you need depends on your career stage:
Career Advancement
Your resume needs to prove trial coordination capability, regulatory knowledge, and staff training experience.
Questions We Ask in Your Interview:
- What trials have you coordinated?
- What regulatory protocols do you know?
What We Highlight on Your Resume:
- Led clinical research trials with recruitment strategies
- Site initiation visits and staff training
- Principal Investigator collaboration
Manager Advancement
Your resume needs to demonstrate multi-study leadership, SOP development, and research centre management.
Questions We Ask in Your Interview:
- What multiple studies have you managed simultaneously?
- What SOPs have you developed?
What We Highlight on Your Resume:
- Multiple BA/BE and Phase I-IV studies simultaneously
- SOPs to validate clinical trial processes
- Led Frontier Clinical Research Centre from scratch
How Do You Write a Clinical Research Coordinator Resume That Gets Interviews?
To write a clinical research coordinator resume that gets interviews, focus on four key sections:
- Professional Summary — highlighting your experience level and specialty areas
- Skills Section — matching keywords from your target job postings
- Work Experience — quantified achievements using the Problem-Solution-Result format
- Credentials — relevant certifications and education
Most "how to write a resume" guides give you generic templates. We interview you to extract specific achievements. Here's what we focus on for Clinical Research Coordinators:
What Should a Clinical Research Coordinator Put in Their Summary?
Your summary must establish both clinical expertise and leadership capability. "End-to-end" shows complete trial ownership. "All trial phases" validates comprehensive experience. Multi-stakeholder collaboration shows communication range. Regulatory compliance focus establishes credibility. Scientific team leadership positions for advancement.
Include professional identity (accomplished and results-oriented professional), experience scope (7+ years of experience steering clinical trials and studies from end-to-end), track record (success mobilizing research personnel throughout all trial phases), stakeholder collaboration (Sponsors, Coordinators, Principal Investigators and Project Managers), leadership approach (dedication to leading, mentoring, and supporting cross-functional teams), and compliance focus (ensuring regulatory compliance, driving scientific teams).
For coordinator positions:
Expert Questions We Ask:
- "What trials have you coordinated?"
- "What stakeholders have you worked with?"
For manager positions:
Expert Questions We Ask:
- "What multiple studies have you led?"
- "What research capabilities have you built?"
What Skills Should Clinical Research Coordinators Highlight?
Your skills must show both regulatory knowledge and operational capability. GCP and ICH knowledge is foundational—list prominently. PMP Certification validates project management. SOP Development shows process leadership. Ethics Board Collaboration shows compliance focus. Risk Assessment shows safety awareness.
Balance certifications (PMP Certification, Extensive knowledge of GCP and ICH) with technical skills (Documentation Review, Database Management, SOP Development & Adherence) and leadership skills (Project Coordination, Staff Training & Supervision, Site Management). Include compliance skills (Policy & Protocol Compliance, Ethics Board Collaboration, Risk Assessment).
Technical skills establish credibility:
Expert Questions We Ask:
- "What GCP/ICH knowledge do you have?"
- "What documentation have you reviewed?"
Leadership skills enable advancement:
Expert Questions We Ask:
- "What staff have you trained?"
- "What sites have you managed?"
How Should Clinical Research Coordinators Structure Experience?
CRC experience must show both trial breadth and regulatory depth. Overview establishes trial phases and stakeholder relationships. Selected Accomplishments with SOP development and multi-study management quantify capability. Key Responsibilities organized by function enables quick scanning. Progressive titles show career advancement.
Include title, company, and dates (Lead Clinical Research Coordinator | Company | Location, 2020-Present). Write Overview establishing trial scope (BA/BE and Phase I-IV clinical trials, in-house trial management, Principal Investigator liaison). Create Selected Accomplishments with specific achievements (SOPs developed, multiple studies simultaneously). Organize Key Responsibilities by function (Project Management, Trial Development Lifecycle, Scope Development, Database Management, Ethics Board Collaboration).
Show trial coordination:
Expert Questions We Ask:
- "What recruitment strategies have you led?"
- "What site initiation visits have you conducted?"
Demonstrate leadership capability:
Expert Questions We Ask:
- "What SOPs have you developed?"
- "What research centres have you led?"
What Education and Certifications Matter for CRCs?
For Clinical Research Coordinators, certifications often matter as much as degrees. PMP Certification validates project management capability essential for trial coordination. Biology or related science degree provides foundation. GCP training demonstrates regulatory knowledge. CTMS proficiency shows technical capability.
Include certifications prominently (PMP Certification with year). List degree with major (Bachelor of Science, Biology). Add clinical research-specific training (CCRC if held, GCP training, CTMS certifications).
Education establishes foundation:
Expert Questions We Ask:
- "What science degree do you hold?"
- "What GCP training have you completed?"
Advanced certifications support advancement:
Expert Questions We Ask:
- "Do you have PMP certification?"
- "What CCRC or similar certifications do you hold?"
Skip the guesswork — let our expert resume writers ask these questions for you.
Schedule Your Resume InterviewHow Does a Resume Interview Extract
Your Clinical Research Coordinator Achievements?
A professional resume interview extracts clinical research coordinator achievements by probing into specific projects, uncovering the goals you were trying to achieve, documenting the systems and processes you implemented, and surfacing challenges you overcame.
What Projects Should You Include
on a Clinical Research Coordinator Resume?
Include projects that demonstrate scope, stakes, and significance. We probe to understand the project value, team size, and your specific role.
How Do You Show Business Impact
on a Resume?
Connect your work to business outcomes by documenting the company's objectives and how your contributions achieved them.
What Systems and Processes
Should You Highlight?
Document the specific systems, processes, and strategies you implemented. This is where your expertise becomes visible.
How Do You Present
Challenges Overcome?
Describe challenges you faced and how you solved them. Problem-solving examples prove you can handle obstacles.
The Power of a 1-on-1 Resume Interview
No cookie-cutter calls. Your interview length matches your career complexity. We ask the questions you can't ask yourself.
Telephone Interview
Telephone Interview
Telephone Interview
Telephone Interview
Telephone Interview
How Competitive Is the
Clinical Research Coordinator Job Market?
Clinical Research Coordinator jobs are highly competitive, averaging 74 applicants per position. With most job seekers applying to 20+ roles, you're competing against approximately 1,480 candidates for the same jobs.
Clinical Research Coordinator Job
Jobs Posted (30 Days)
Per 20 Applications
Hardest to Land
Most competitive pharmaceutical rolesEasier to Land
Less competitive pharmaceutical rolesData based on LinkedIn job postings, updated January 2026. View full job market data →
Here's the math most job seekers don't do:
Your resume needs to stand out against 1,480 other pharmaceutical professionals.
Most of them list the same projects. The same certifications. The same responsibilities.
What makes you different is the story behind the projects.
Pharmaceutical Professionals We've Helped Are Now Working At
From general contractors to specialty trades, our clients land roles at top pharmaceutical firms across North America.
Reach Pharmaceutical's Hidden Job Market
80% of pharmaceutical positions are never advertised. Get your resume directly into the hands of recruiters filling confidential searches.
Pharmaceutical Recruiter Network
When you purchase our Resume Distribution service, your resume goes to 200+ recruiters specializing in pharmaceutical — included in Advanced & Ultimate packages.
Clinical Research Recruiters
Boston, MA
Pharma Talent Partners
San Diego, CA
Sample Pharmaceutical Recruiters
200+ Total| Agency | Location |
|---|---|
CRR Clinical Research Recruiters |
Boston, MA |
PTP Pharma Talent Partners |
San Diego, CA |
LSS Life Sciences Staffing |
Research Triangle, NC |
Frequently Asked Questions About
Clinical Research Coordinator Resumes
A strong Clinical Research Coordinator resume should highlight trial phase experience (Phase I-IV, BA/BE studies), regulatory knowledge (GCP, ICH, SOPs), Ethics Board collaboration (informed consent, ethics documentation), multi-study management (simultaneous trials), and Key Responsibilities organized by function (Trial Development Lifecycle, Database Management, Site Management, Regulatory Compliance). Include certifications prominently.
Specify all phases managed: "BA/BE and Phase I-IV studies." Show simultaneous capability: "multiple studies simultaneously." Include therapeutic areas: "allergy research," "dentistry," "endocrinology." Note trial types: "multi-center clinical trials," "in-house trial management." Phase breadth demonstrates comprehensive clinical research capability.
PMP Certification validates project management capability for trial coordination. GCP/ICH knowledge is essential—highlight extensively. CCRC (Certified Clinical Research Coordinator) is valuable if held. Bachelor of Science in Biology or related field provides foundation. Include CTMS proficiency and other clinical trial management systems.
Document protocol adherence: "manages staff compliance to established regulatory protocols and SOPs." Show Ethics Board work: "prepares ethics documentation for board review and approval." Include risk mitigation: "mitigate potential health, safety, and ethical risks." Note informed consent: "create informed consent forms." Regulatory focus is essential for CRC credibility.
Organize by trial function: Project Management & Leadership (goals, assignments, schedule), Trial Development Lifecycle (concept to execution, trial master file), Scope Development (resource requirements, protocols), Database Management & Progress Tracking (CTMS, deviations), Report Generation (sponsor reporting), Resource Estimation & Vendor Management, Site Management, Regulatory Compliance Management, Ethics Board Collaboration, Equipment Maintenance.
Absolutely—centre leadership differentiates senior candidates. Document building capability: "developing trial strategies from scratch." Show template creation: "designed research and data templates." Include training role: "advising and coaching students and professors." Note academic collaboration: "University Ethics Board," "dentists, orthodontists and graduate students." Research centre experience positions for management roles.
Ready to Transform Your Resume?
Schedule your 60-minute interview and get a resume that proves you're the obvious choice.
Choose Your Interview LengthHave Questions?
Talk to an advisor who can recommend the right package for your situation.
Talk to an Advisor 1-877-777-6805