Clinical Research Associate
Resume Sample
A real resume example showing how we transform CRA expertise into proof sponsors and CROs trust
Being qualified isn't enough — you need to be the obvious choice.
We fix your resume with one conversation
What Makes a Strong Clinical Research Associate - CRA Resume?
A Clinical Research Associate resume must prove ability to monitor clinical trials, ensure regulatory compliance, and maintain data integrity. Sponsors and CROs scan for GCP certification, therapeutic area experience, and multi-site monitoring capability. This sample demonstrates Post Graduate Diploma in Clinical Research with GCP certification, monitoring experience including pre-study visits, site initiation, interim visits, and close-out, regulatory expertise across FDA, Health Canada, EMEA, and ICH guidelines, therapeutic experience in oncology, neurology, dermatology, and gastrointestinal disorders, and pharmacovigilance and fraud/misconduct reporting capability.
Why Do Clinical Research Associate - CRA Resumes
Get Rejected?
Most clinical research associate - cra resumes get rejected not because of ATS software, but because they don't prove you're better than the other 51 applicants. Generic bullets like "managed construction projects" don't differentiate you — quantified achievements do.
See how we transform generic statements into interview-winning proof:
We showed monitoring breadth (evaluation, initiation, maintenance, close-out), compliance framework (GCP, SOPs), and documentation scope (CRFs, reports, archives). Complete visit cycle experience demonstrates senior capability.
We showed therapeutic diversity (GI, dermatology, neurology, oncology), specific indications (ulcerative proctitis, onychomycosis, epilepsy), and regulatory breadth (FDA, Health Canada, EMEA, ICH). Multiple therapeutic areas with specific indications proves clinical depth.
We showed protocol development (develop and write), site feasibility assessment, investigator liaison, and regulatory applications management (IND, NDA, TPD). Protocol writing plus regulatory submissions demonstrates senior CRA capability.
How Do Pharmaceutical Resume Writers Transform a Clinical Research Associate - CRA Resume?
Professional resume writers transform clinical research associate - cra resumes by analyzing job postings for required keywords, extracting specific achievements through targeted questions, quantifying impact with dollar values and percentages, and positioning you as the solution to employer problems.
We Analyze Clinical Research Associate - CRA Job Postings
We identify exactly what hiring managers search for:
- Budget management and cost control requirements
- Schedule recovery and timeline management skills
- Site safety compliance and OSHA standards
- Subcontractor coordination and vendor management
We Extract Your Achievements
Our 1-on-1 interview uncovers:
- Project values and budgets you've managed
- Team sizes and subcontractors you've coordinated
- Problems you've solved that others couldn't
- Metrics you didn't think to track or quantify
We Quantify Your Impact
We find the numbers that prove ROI:
- Dollar values of projects completed on time
- Percentage of schedule improvements achieved
- Cost savings from value engineering decisions
- Safety record improvements and incident reductions
We Position You as the Solution
Your resume proves you solve employer problems:
- Delivering projects on time despite site challenges
- Managing subcontractors and maintaining quality
- Controlling costs while meeting specifications
- Leading teams through complex project phases
Listen to a Real Resume Interview
Hear how our writers extract monitoring achievements from clinical research professionals.
What Does a Clinical Research Associate - CRA Resume Interview Look Like?
A clinical research associate - cra resume interview is a conversation where our writer asks targeted questions about your projects, probes for specific details, and extracts achievements you'd never think to include.
Experienced in monitoring multiple site locations, clinical site evaluation, initiation, maintenance and close-out visits in accordance with GCP and company SOP's, while ensuring integrity of study data and following study timelines. Verify data on CRFs; monitor schedules; file and collate trial documentation and reports; archive study documentation and correspondence; preparing final reports.
Every bullet on this resume was created through this same process.
Schedule Your InterviewHave questions? 1-877-777-6805
Watch How We Transformed This Clinical Research Resume
See how our interview process uncovered regulatory expertise and therapeutic experience.
Get Your Resume Transformed
What a Clinical Research Associate - CRA Resume Example That Gets Interviews Looks Like
A complete clinical research associate - cra resume is typically 1-2 pages and includes a professional summary, core competencies, detailed work experience with quantified achievements, education, and certifications. Here's an actual resume created through our interview process.
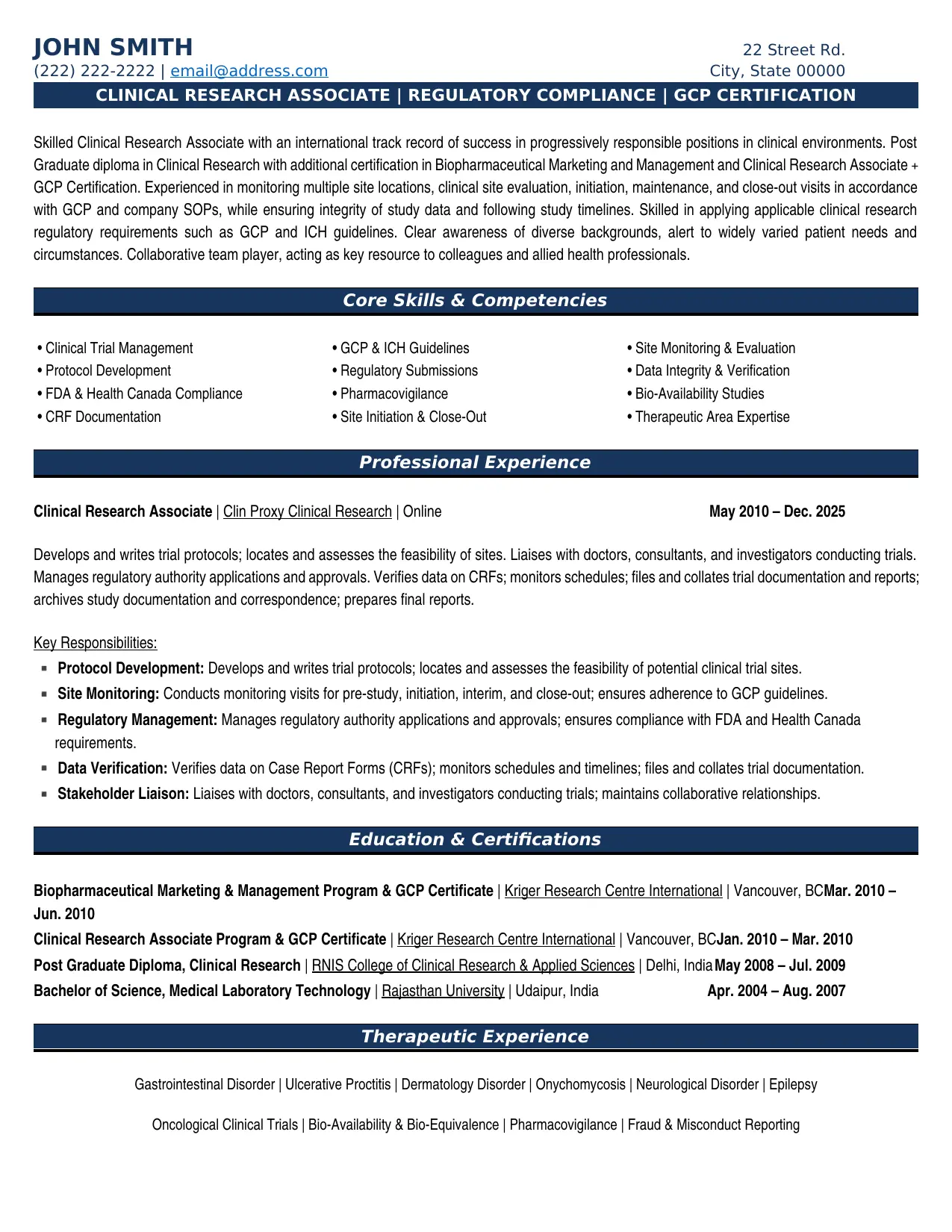
Which Clinical Research Associate - CRA Resume Example
Do You Need?
The clinical research associate - cra resume you need depends on your career stage:
Senior CRA or Lead CRA Position
Your resume needs to prove monitoring capability, GCP knowledge, and regulatory compliance experience.
Questions We Ask in Your Interview:
- What monitoring visits have you conducted?
- What therapeutic areas have you worked in?
What We Highlight on Your Resume:
- Site monitoring visit types conducted
- Therapeutic area diversity
Clinical Trial Manager or Project Manager Transition
Your resume needs to differentiate you through multi-site management, protocol development, and regulatory authority interactions.
Questions We Ask in Your Interview:
- How many sites have you monitored?
- What protocol development have you done?
What We Highlight on Your Resume:
- Multiple site location monitoring
- Protocol development and regulatory applications
How Do You Write a Clinical Research Associate - CRA Resume That Gets Interviews?
To write a clinical research associate - cra resume that gets interviews, focus on four key sections:
- Professional Summary — highlighting your experience level and specialty areas
- Skills Section — matching keywords from your target job postings
- Work Experience — quantified achievements using the Problem-Solution-Result format
- Credentials — relevant certifications and education
Most CRA resumes list monitoring responsibilities without demonstrating therapeutic diversity or regulatory depth. Our interview process extracts the specific indications, protocol development, and multi-agency regulatory knowledge that prove you can manage complex trials.
What Should a Clinical Research Associate Put in Their Profile Summary?
Your summary must signal both technical capability and professional maturity. This CRA describes "international track record of success" with "Post Graduate diploma in Clinical Research" and "GCP Certification," experienced in "monitoring multiple site locations" while "ensuring integrity of study data."
Lead with international track record and credentials. Highlight GCP certification and monitoring experience. Emphasize data integrity, regulatory compliance, and patient-centered awareness. Show collaborative approach and quality improvement focus.
For coordinators or junior CRAs seeking senior roles...
Expert Questions We Ask:
- "What monitoring visits have you conducted?"
- "What therapeutic areas have you worked in?"
For CRAs targeting CTM or project manager roles...
Expert Questions We Ask:
- "How many sites have you monitored?"
- "What protocol development have you done?"
What Skills Should a Clinical Research Associate Highlight?
Skills must show both regulatory depth and clinical breadth. This resume includes IND/NDA applications alongside therapeutic experience in oncology, neurology, dermatology — demonstrating ability to support trials from regulatory submission through monitoring.
Include regulatory, monitoring, and therapeutic expertise: Ethics in Clinical Research, ICH Guidelines, GCP, FDA/Health Canada/EMEA regulations alongside Monitoring visit types, Bio-availability/Bio-Equivalence Guidelines, Pharmacovigilance. List specific therapeutic areas with indications.
GCP and monitoring skills establish foundation...
Expert Questions We Ask:
- "What GCP training have you completed?"
- "What visit types can you conduct?"
Regulatory submissions and therapeutic diversity differentiate...
Expert Questions We Ask:
- "What regulatory submissions have you supported?"
- "How many therapeutic areas?"
How Should a Clinical Research Associate Describe Their Experience?
Every responsibility must connect to trial quality or compliance. This resume shows "monitoring multiple site locations" with "GCP and company SOP's," "verify data on CRFs," "manage regulatory authority applications," and "develop and write trial protocols."
Lead with monitoring scope and compliance framework. Include protocol development, site feasibility, investigator liaison, and regulatory applications. Emphasize CRF verification, documentation management, and report preparation.
Show monitoring fundamentals and documentation skills...
Expert Questions We Ask:
- "What visits have you conducted?"
- "What documentation have you managed?"
Demonstrate protocol development and regulatory liaison...
Expert Questions We Ask:
- "What protocols have you written?"
- "What regulatory submissions have you managed?"
What Credentials Matter for Clinical Research Associates?
Credentials should show both clinical research training and scientific foundation. This resume shows Post Graduate Diploma in Clinical Research, GCP Certificate, Clinical Research Associate Program Certificate, plus Bachelor of Science in Medical Laboratory Technology.
Include clinical research diplomas and GCP certification prominently. List specialized certificates in biopharmaceutical marketing, management, or therapeutic areas. Undergraduate degree in life sciences provides foundation.
GCP certification and CRA training establish eligibility...
Expert Questions We Ask:
- "Do you have GCP certification?"
- "What clinical research training?"
Advanced certifications and management training differentiate...
Expert Questions We Ask:
- "What specialized certifications do you have?"
- "What management training?"
Skip the guesswork — let our expert resume writers ask these questions for you.
Schedule Your Resume InterviewHow Does a Resume Interview Extract
Your Clinical Research Associate - CRA Achievements?
A professional resume interview extracts clinical research associate - cra achievements by probing into specific projects, uncovering the goals you were trying to achieve, documenting the systems and processes you implemented, and surfacing challenges you overcame.
What Projects Should You Include
on a Clinical Research Associate - CRA Resume?
Include projects that demonstrate scope, stakes, and significance. We probe to understand the project value, team size, and your specific role.
How Do You Show Business Impact
on a Resume?
Connect your work to business outcomes by documenting the company's objectives and how your contributions achieved them.
What Systems and Processes
Should You Highlight?
Document the specific systems, processes, and strategies you implemented. This is where your expertise becomes visible.
How Do You Present
Challenges Overcome?
Describe challenges you faced and how you solved them. Problem-solving examples prove you can handle obstacles.
The Power of a 1-on-1 Resume Interview
No cookie-cutter calls. Your interview length matches your career complexity. We ask the questions you can't ask yourself.
Telephone Interview
- Students / New Grads
- Specialists, Analysts, Coordinators
- Targeting mid-level positions
Telephone Interview
- Individual Contributors
- Managers
- Career Changers
- Seeking Promotions
- Masters / Ph.D Holders
Telephone Interview
- Senior Managers
- Directors
- Department Heads
- Senior Writer Assigned
Telephone Interview
- Vice Presidents
- C-Suite Executives
- Business Owners
- Senior Writer Assigned
- Executive Resume Format
How Competitive Is the
Clinical Research Associate - CRA Job Market?
Clinical Research Associate - CRA jobs are medly competitive, averaging 52 applicants per position. With most job seekers applying to 20+ roles, you're competing against approximately 1,040 candidates for the same jobs.
Clinical Research Associate - CRA Job
Jobs Posted (30 Days)
Per 20 Applications
Hardest to Land
Most competitive pharmaceutical rolesEasier to Land
Less competitive pharmaceutical rolesData based on LinkedIn job postings, updated February 2026. View full job market data →
Here's the math most job seekers don't do:
Your resume needs to stand out against 1,040 other pharmaceutical professionals.
Most of them list the same projects. The same certifications. The same responsibilities.
What makes you different is the story behind the projects.
Pharmaceutical Professionals We've Helped Are Now Working At
From general contractors to specialty trades, our clients land roles at top pharmaceutical firms across North America.
Reach Pharmaceutical's Hidden Job Market
80% of pharmaceutical positions are never advertised. Get your resume directly into the hands of recruiters filling confidential searches.
Pharmaceutical Recruiter Network
When you purchase our Resume Distribution service, your resume goes to 280+ recruiters specializing in pharmaceutical — included in Advanced & Ultimate packages.
EPM Scientific
New York, NY
Proclinical
Philadelphia, PA
Sample Pharmaceutical Recruiters
280+ Total| Agency | Location |
|---|---|
EP EPM Scientific |
New York, NY |
PC Proclinical |
Philadelphia, PA |
KS Kelly Science |
Boston, MA |
Frequently Asked Questions About
Clinical Research Associate - CRA Resumes
Your resume must demonstrate GCP certification, monitoring experience, and therapeutic area expertise. Include visit types conducted (this sample shows evaluation, initiation, maintenance, close-out), regulatory knowledge (FDA, Health Canada, EMEA, ICH), therapeutic areas (oncology, neurology, dermatology, GI), and documentation skills (CRF verification, protocol development).
The CRA market shows 52 applicants per position. Competition is moderate with emphasis on therapeutic area experience. Candidates with oncology trials, GCP certification, and multi-site monitoring experience differentiate significantly.
Include regulatory and monitoring skills: Good Clinical Practice (GCP), ICH Guidelines, FDA and Health Canada regulations alongside Monitoring-Pre-study Visit, Site Initiation, Interim Visit & Site Close-Out, Bio-availability and Bio-Equivalence Guidelines, Pharmacovigilance.
List therapeutic areas with specific indications. This resume shows "Gastrointestinal Disorder, Ulcerative Proctitis; Dermatology Disorder, Onychomycosis; Neurological Disorder, Epilepsy; Oncological Clinical Trials." Specific indications prove deeper clinical knowledge than generic therapeutic areas.
Absolutely — protocol work demonstrates senior capability. This resume shows "Develop and write trial protocols; locate and assess the feasibility of sites" plus "Manage regulatory authority applications and approvals." Protocol development plus regulatory submissions prove advancement readiness.
Include GCP certification and clinical research credentials. This resume shows Post Graduate Diploma in Clinical Research, GCP Certificate, Clinical Research Associate Program Certificate, Biopharmaceutical Marketing & Management Certificate. Multiple credentials demonstrate comprehensive preparation.
Ready to Transform Your Resume?
Schedule your 45-minute interview and get a resume that proves you're the obvious choice.
Choose Your Interview LengthHave Questions?
Talk to an advisor who can recommend the right package for your situation.
Talk to an Advisor 1-877-777-6805